Business
LMS
THE TECHNOLOGY!

- Business
- Bio
Bio
Globally Certified Bio Single-Use Bags & System
With cutting-edge production environments and quality systems, our bio division manufactures single-use bags and systems that are essential for biopharmaceutical processes.
01 Product Overview
Single-Use Bags
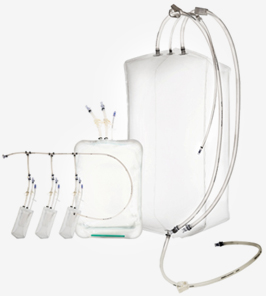
Assembly
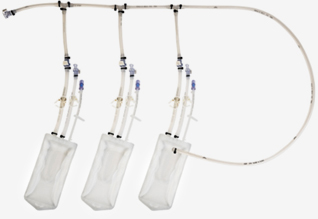
-
01Our portfolio covers a broad range of products, from 2D, 3D, to liner bags.
-
02We specialize in producing single-use bags and systems for bioprocessing.
-
03Prompt response to both standard and custom product requests.
02 Product Lineup
BioCELVAS 2D Bags
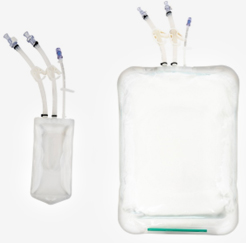
- Sampling
- 50ml, 100ml, 200ml, 500ml, 1L, 2L
- Storage
- 5L, 10L, 20L, 50L
BioCELVAS 3D Bags

- Storage
- 50L, 100L, 200L, 250L, 500L, 1,000L
BioCELVAS Liner Bags
- Storage
- 20L, 50L, 100L, 200L, 300L, 370L, 560L
03 Application
| Application | Bag Types Used |
|---|---|
| Media & Buffer preparation | Liner bags, 2D bags, 3D bags |
| Storage of cell fluid, intermediate products, final products | 2D bags, 3D bags |
| Sampling | 2D bags |
| Shipping & Transportation | 2D bags, 3D bags |
| Wastes Collection | Liner bags |
04 Manufacturing Environment and Facility

ISO Class 7 Cleanroom
- - Each process is carried out in a separate, dedicated workspace to minimize
the risk of cross-contamination. - - GMP compliant environment
- - Provide high quality production

Equipped with cutting edge helium leak tester
05 Quality System and Certification
Key Features
01
ISO 13485:2016
Certification
02
Products produced in
ISO class 7 cleanroom
03
Meets global standards -
ISO, USP, EP including FDA class VI
04
Extractables test performed
according to BPOG protocol
05
Bacterial endotoxin
study performed
06
Delivery after 100%
helium leak test
07
Delivery after 100%
Visual Inspection
08
Sterilized by gamma
irradiation
Certification and TEST report
ISO 13485 Certification
Quality System Conforms to ISO 13485 Standard

Extractables test Report
(according to BPOG protocol)

